Transparent, Protected and as Cold as Ice
SCHREINER PROSECURE
Transparent, Protected and as Cold as Ice
Ever since the various COVID-19 vaccines have been discussed in public, the fact that some pharmaceuticals require refrigeration at extremely low temperatures has become common knowledge. This is true not only in the context of combatting the corona pandemic but also in many other areas, where deep-frozen and cryo-products call for tamper-proof packaging. However, up until now, the selection of materials that can be used for realizing closure seals for such applications has been limited—and practically all of them are opaque. The Schreiner ProSecure competence center in close cooperation with the pharma experts from the Schreiner MediPharm business unit has now succeeded in developing a portfolio of counterfeit-proof and tamper-proof closure seals for low-temperature and cryogenic applications.
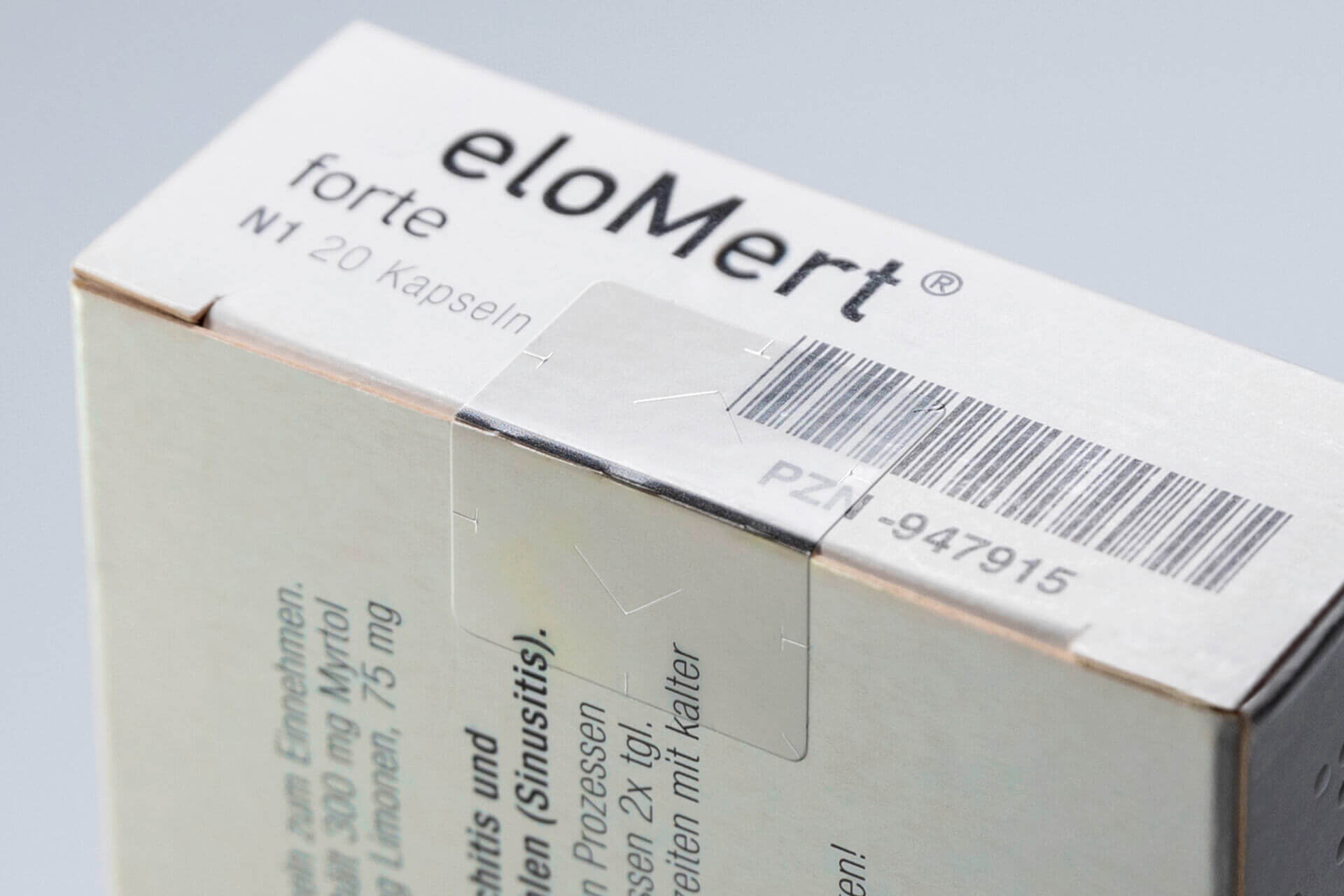
Transparency is far more than merely a gimmick. In everyday life, we encounter countless examples of objects where transparency is crucial, from windshields and windows to clear plastic folders. Transparent closure seals are particularly essential for secondary pharmaceutical packaging to prevent key medication information from being concealed by the application of a seal. Even so, tamper-proof seals used for frozen products, such as gene therapy medications, have typically been opaque until now.
Due to the utilization of new transparent materials for use with frozen products and extension of the materials portfolio by novel types of transfer adhesives, Schreiner ProSecure is now able to achieve transparent closure seals also for low-temperature and cryogenic applications. As prescribed, the face materials are easily destructible and clearly provide counterfeit and tamper protection. Of course, the closure seals comply with the requirements of the European Falsified Medicines Directive 2011/62/EU. Thanks to this extension of the materials portfolio by Schreiner ProSecure, Schreiner MediPharm is now in an even better position to respond to the special needs of its pharmaceutical customers.








