Smart Helpers in Hospitals: Prefilled Syringes with RFID
SCHREINER MEDIPHARM
Smart Helpers in Hospitals: Prefilled Syringes with RFID
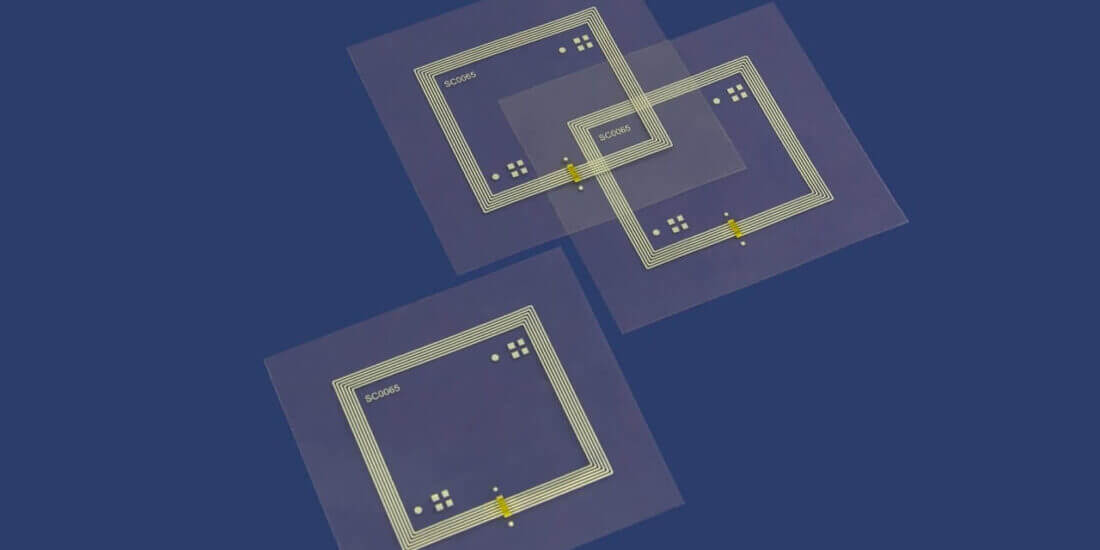
Automated, intelligent, and connected: Digitization is finding its way into modern hospitals and is suitable for a wide variety of uses—such as in inventory management, patient care and documentation, identification of medications and medical devices, and in the entire management system. The key lies in RFID technology. It converts conventional marking labels into smart labels that optimize processes, enhance product safety, and help avoid medication errors. Schreiner MediPharm and SCHOTT Pharma offer a coordinated, newly developed solution for equipping prefilled syringes with RFID. The combination of syringe and smart label opens up a wide range of optimization opportunities for everyday hospital settings. However, equipping the syringes with RFID entails many challenges to be considered in the development process.
The partnership between Schreiner MediPharm and SCHOTT Pharma has previously been focused primarily on equipping COC syringes with analog functional labels. Now the two pharmaceutical packaging experts are taking another promising step by digitizing prefilled syringes. What makes the new RFID Syringe Labels special is the combination of marking the syringe with its unique, digital identity. In addition, digital first-opening indication to protect the integrity of the syringe is possible. To successfully implement this innovation and to ensure impeccable RFID functionality in terms of good performance and adequate range, various characteristics of the prefilled syringe must be considered:
Material
Syringes can be made of diverse materials such as COC, PP, or glass. These materials may affect range and trouble-free reading of the tag.
Size and Diameter
The smaller the syringe the less space for product marking and integration of the RFID chip. Plus, the smaller the tag the shorter usually its range. In addition, especially in the case of small syringe diameters, the curvature may affect performance. Besides that, most syringe labels have transparent areas for visual inspection of the syringe content and the graduation, which further reduces the space available for the inlay.
Active Ingredient, Quantity, and Fill Level
Medications are composed of diverse substances and differ in terms of their dielectric properties. Especially water-based active ingredients have a negative impact on the radio transmission performance of a tag. Consequently, the position of the RFID-Label and the integrated inlay must be adapted to the liquid and fill level. In bulk reading processes, containers and liquids of other products may affect readability as well.
Aside from these syringe characteristics, the utilization of RFID requires special data standards for identification and tracking on unit level, such as UnitVisID or GS1. They enable all parties involved to interpret and use the data. There are two variants:
1. Offline
Product name, dose, expiration date, batch number, etc. are stored directly on the tag and can be read and interpreted by any compatible device.
2. Online
Only an identification number (ID) is stored on the tag. All product-related data are queried in an online database.
Variant 1 requires no online connection of the infrastructure but more storage space, plus the tags are more cost-intensive. Encoding of the tags during the manufacturing process requires more time, which increases with the volume of data to be encoded. Whether the offline or online variant is more ideally suited as the system solution must be analyzed for the specific use case. The same applies to the choice of reading system that can be implemented using either stationary devices or handheld readers.
Making Pre-filled Syringes Smart
The new solution from Schreiner MediPharm and SCHOTT Pharma was presented for the first time in October 2022 at the “PDA Universe of Pre-filled Syringes and Injection Devices Conference” in the United States. Arne Rehm, Senior Product Manager RFID/NFC Solutions at Schreiner MediPharm, and Tom van Ginneken, Head of Global Product Management for SCHOTT TOPPAC®, delivered a joint presentation under the motto “Making Pre-filled Syringes Smart” at the event.