Optimal Protection for Syringes: Digitalization and Efficiency Increase
SCHREINER MEDIPHARM
Optimal Protection for Syringes: Digitalization and Efficiency Increase
Digital solutions are key for enhanced inventory management and patient safety in hospital environments. At the same time, product integrity has to be ensured until the point of use. Schreiner MediPharm’s Cap-Lock Label with RFID functionality enables digital tracking of prefilled syringes on unit level as well as enhanced efficiencies along the supply chain.
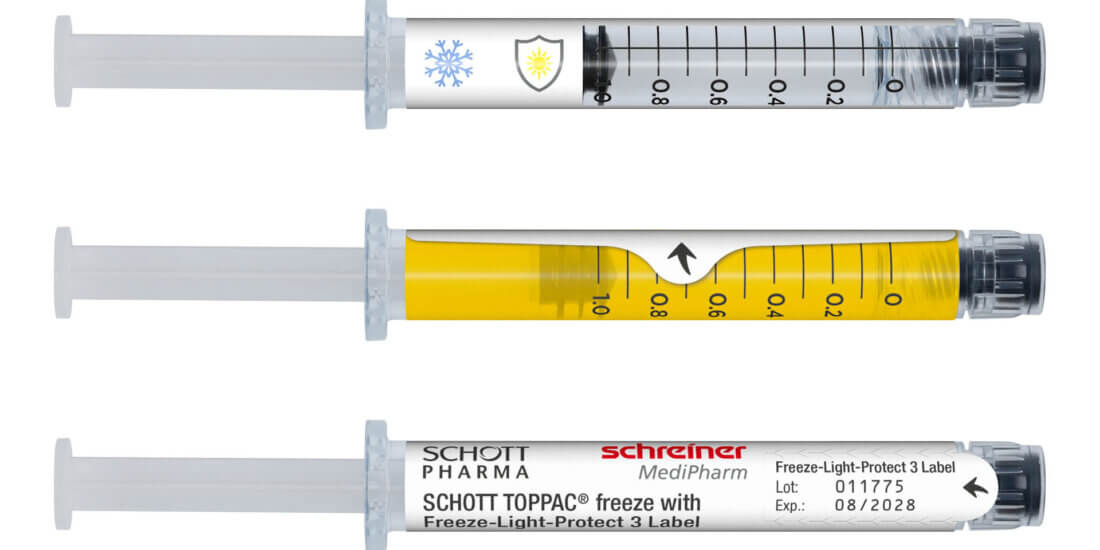
The new Cap-Lock plus RFID is based on the proven principle of Schreiner MediPharm’s Cap-Lock Label and is combined with SCHOTT Pharma’s SCHOTT TOPPAC® infuse COC syringes. Thanks to a special cap design that has the same diameter as the syringe barrel, the Cap-Lock Label wraps around the syringe and cap. A label-integrated irreversible first-opening indication is activated as soon as the cap is opened. The solution safeguards the integrity of the prefilled syringe until final use, thus replacing the usual blister pack by a sustainable alternative enabling considerable CO₂ reductions and cost savings. Additionally, due to optimized packaging and distribution processes as well as reduced space requirements during transportation and storage, it helps enhance efficiencies along the entire supply chain.
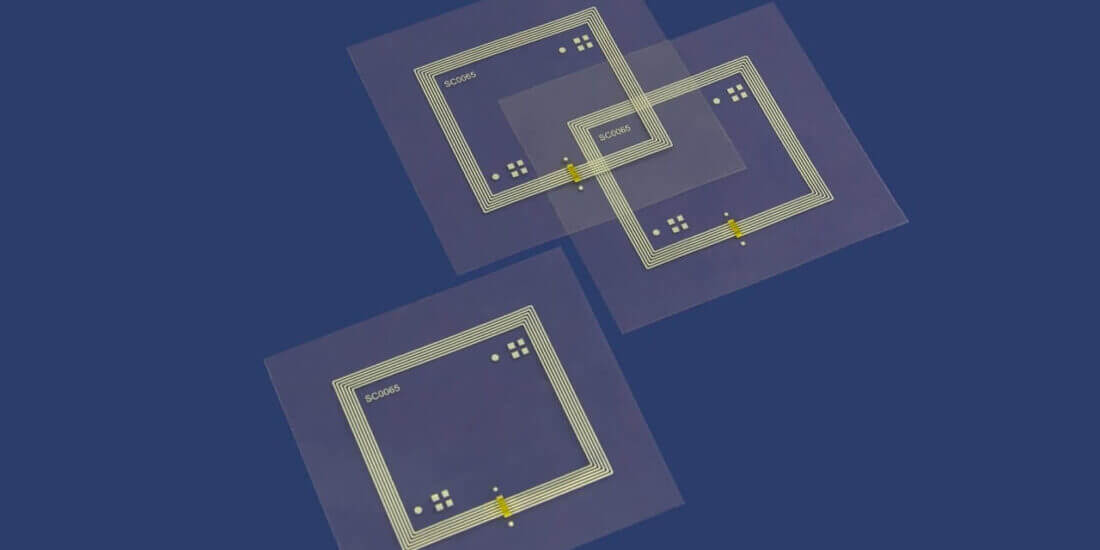
The special innovation of Schreiner MediPharm’s Cap-Lock plus RFID is its integrated RFID functionality. In addition to the analog first-opening indication, the chip embedded in the label offers a digital first-opening indication. In hospital settings, this enables automated tracking of the integrity of each individual prefilled syringe—up until the medication is administered. Consequently, there is no need to manually check if the syringe has previously been opened, resulting in optimized and reliable processes for healthcare staff. At the same time, clear identification on unit level is supported, which makes seamless tracking in the supply chain possible and facilitates automated inventory management in daily hospital settings. Expired drugs are automatically detected and unused prefilled syringes can be returned to inventory in a reliable process. Potential diversions and medication misuse can be monitored digitally.
Like all RFID solutions from MediPharm, the new Cap-Lock plus RFID features a particularly robust design. A special label construction reliably protects the integrated chip against mechanical stress and functional failure—even on the high-speed dispensing systems in pharmaceutical manufacturing. Thus, the readability and functional reliability of the label-integrated RFID chip is guaranteed from production to final use. As a result, pharmaceutical companies are provided with a reliable solution avoiding additional costs due to production downtimes and waste rates. At the same time, product and patient safety is enhanced.