New Packaging for Syringes: Secure, Sustainable, Blister-Free
SCHREINER MEDIPHARM
New Packaging for Syringes: Secure, Sustainable, Blister-Free
Schreiner MediPharm together with SCHOTT Pharma and Körber Pharma has developed an innovative packaging concept for prefilled syringes requiring no blister packaging. The new solution offers numerous benefits in terms of sustainability, security, and cost efficiency and met with strong interest at this year’s Pharmapack in Paris. The three partners are thus emphasizing their pioneering role in the field of pharmaceutical packaging.
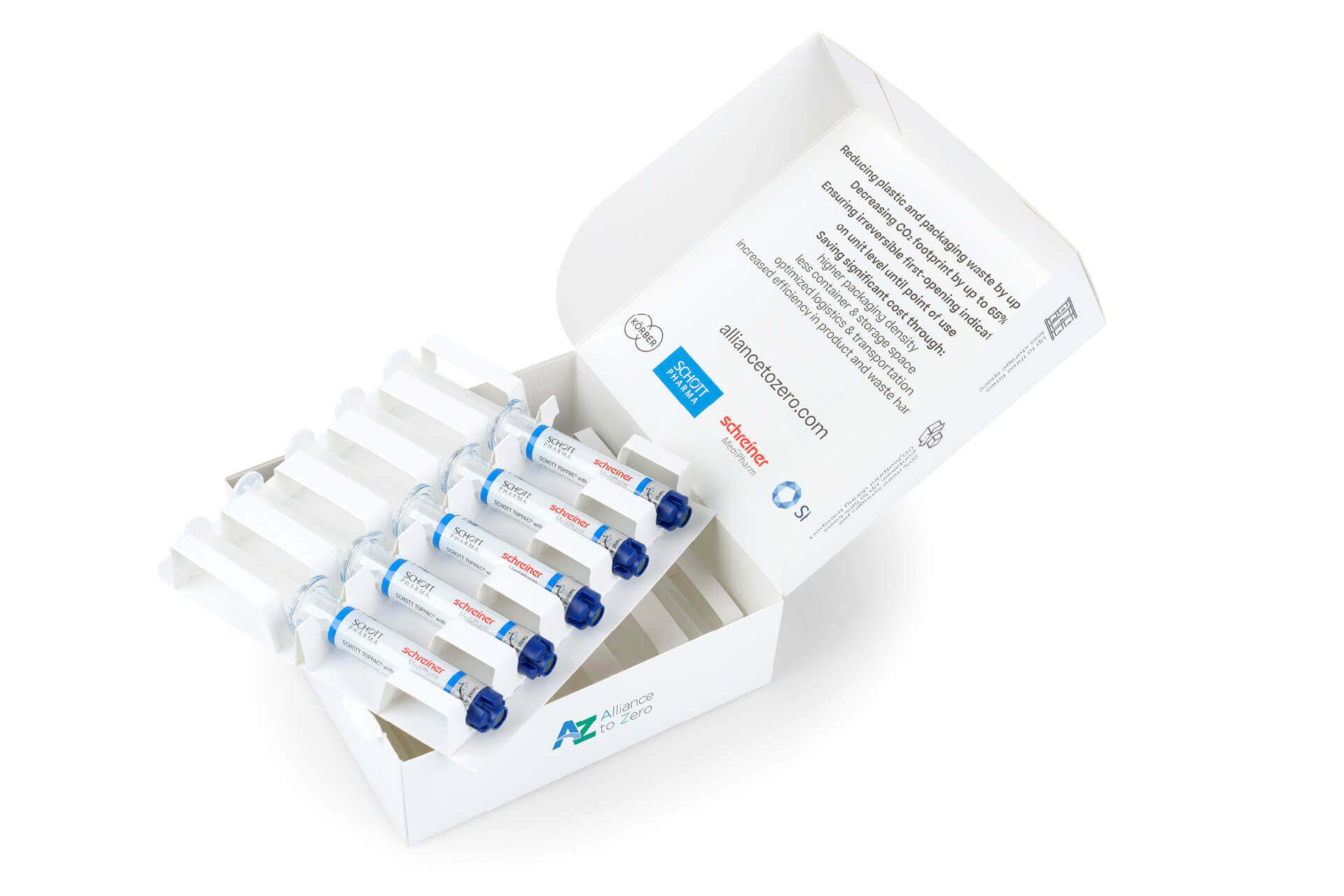
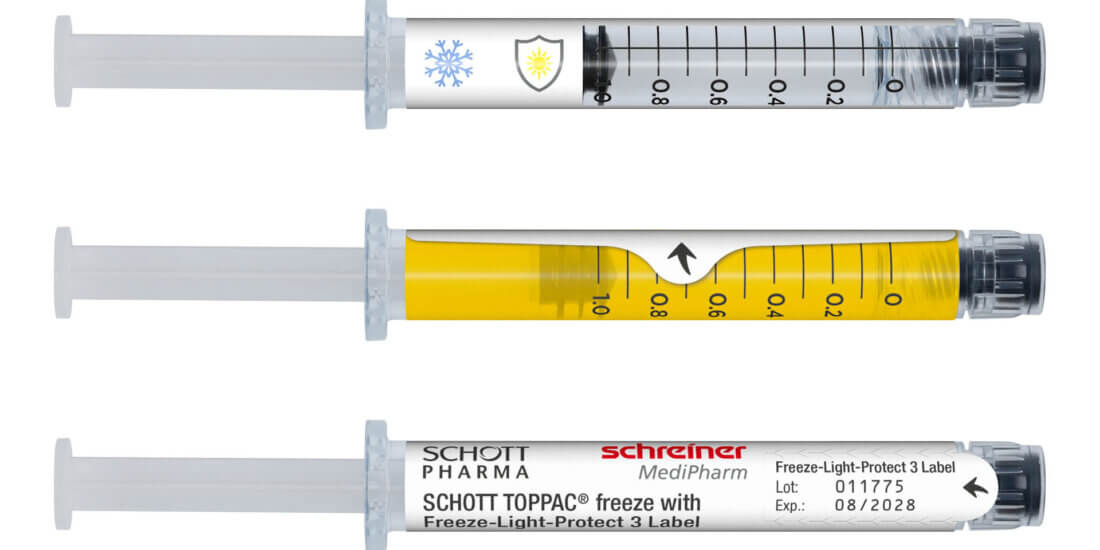
The blister-free concept is based on the combination of a SCHOTT TOPPAC® infuse COC syringe with an innovative specialty cap and the novel Cap-Lock Label from Schreiner MediPharm. Due to the new cap design, which has the same diameter as the syringe barrel, the Cap-Lock Label can be applied to the syringe barrel and cap in a reliable process, enabling tamper-evident first-opening indication. Opening the cap irreversibly damages the label so that users immediately see if the syringe has been opened before. “With this solution, we ensure maximum product safety and integrity—from manufacturing to final use,” explains Dr. Nadine Lampka, Senior Product Manager Pharma-Security at Schreiner MediPharm.
Moreover, the Cap-Lock Label performs other protective functions of the conventional blister and transfers them to the primary container. For instance, it can include a gas barrier and multi-level UV and light protection ensuring the stability of the active ingredients. In addition, the extended label area provides ample space for individual product markings and color codes. The integration of an RFID chip for automated tracking on unit level and for digital first-opening indication are possible as well. The combination of a COC syringe with a special cap and functional label with a seal function enables the utilization of a volume-optimized top-load cardboard box from Körber Pharma consisting of 100 percent mono-material. That optimizes packaging and distribution processes in pharmaceutical manufacturing and clearly reduces the space required for transportation and storage.
The new concept offers considerable economic benefits for pharmaceutical manufacturers—while clearly enhancing sustainability. The space-saving packaging reduces material consumption and enables more efficient logistics. “Due to eliminating the need for blister packaging, we can reduce plastics and plastics waste by up to 80 percent and significantly increase transportation capacity at the same time,” emphasizes Christoph Zauner, Head of Global Product Management for Polymer Solutions at SCHOTT Pharma. A sample calculation shows that the number of containers for one shipment of ten million 5 ml SCHOTT TOPPAC® infuse syringes from Hamburg to New York can be reduced by 16 while avoiding around 87,400 kilograms of CO2 emissions.
At Pharmapack, the three partner firms presented the solution as part of the Alliance to Zero Workshop “How to Build Sustainable Value Chains Together”. Lidia Smith, Sustainability and Product Manager at Körber Pharma, emphasizes, “The great response at the trade fair shows that sustainable packaging concepts are urgently needed. Our collaboration with Schreiner MediPharm and SCHOTT Pharma is an important step toward an eco-friendlier pharma supply chain.” Schreiner MediPharm underscores its commitment to innovative, sustainable solutions with further functional labels for pre-filled syringes including Needle-Trap Secu and Syringe-Closure-Wrap, both of which enable secure, blister-free packaging as well.