Healthcare: RFID/NFC-Trends
SCHREINER MEDIPHARM
Healthcare:
RFID/NFC-Trends
Digital patient health and automated processes in hospital settings are becoming increasingly important. RFID-based solutions assume an important role in that regard. Frederic Vicentini and Sylvia Kaiser-Kershaw from our RFID partner company NXP® Semiconductors and Arne Rehm from Schreiner MediPharm discuss the latest developments.
What are the most important trends with RAIN-RFID and its use for pharmaceutical products?
Frederic Vicentini, NXP (FV): RAIN-RFID, in other words UHF-based RFID technology according to the RAIN industry alliance, offers major opportunities for end-to-end tracking and tracing of drugs and medical consumables on unit level–from production through the supply chain all the way to the point of dispense, for instance in a hospital. It enables easy bulk reading at long range, serialization, and automated, precise inventory management in real time. That helps reduce errors, facilitates recalls, and enables automatic refilling at the hospital. At the same time, the transparency of pharmaceutical products in the entire supply chain is significantly enhanced.
Specific statistics illustrate the benefits. For instance, savings of 50,000 US dollars per year are achieved in a healthcare facility with more than 1,000 beds, according to UnitVisID. The process of replenishing inventory is error-free and about 90 % faster than in manual processes. Thanks to the higher transparency of inventory management, there is less waste and 1-3 % of drug expenditures can be saved.
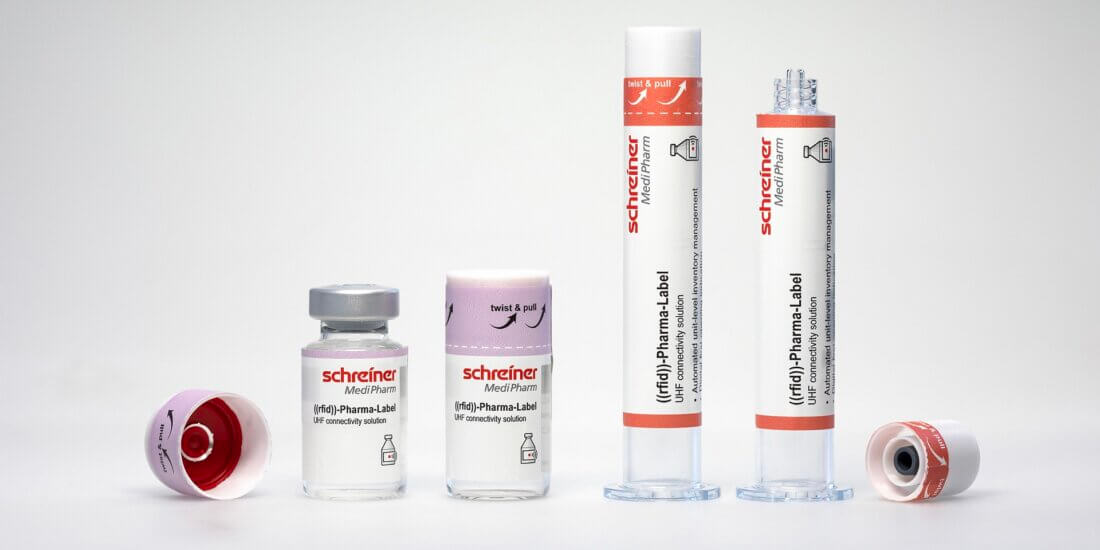
Arne Rehm, Schreiner MediPharm (AR): A major trend is marking products with an RFID label directly in the pharmaceutical manufacturer’s production process (source tagging) instead of manually at the hospital or pharmacy. This contributes significantly to process optimization and enables easier and more efficient use of automated inventory management systems. It enables easy bulk reading of drugs in syringes, vials, or other primary containers in large quantities without an additional process step. Source tagging makes special demands on the label and is enabled primarily by the flexible yet particularly durable construction of our Robust RFID-Label that supports reliable RFID performance from production to final use.
What are the most important drivers for the adoption of NFC in the healthcare sector?
Sylvia Kaiser-Kershaw, NXP (SKK):Generally, there is an increasing trend toward connectivity. Acceptance of smartphones for contactless NFC transactions significantly increased especially during the COVID pandemic. ABI Research predicts a growth in the installed base of NFC-capable devices to 5.9 billion in 2028. As far as the healthcare sector is concerned, the growing trend toward in-home care and self-medication, specifically of biologics, plays a major role. That calls for solutions to enhance patient safety and medication adherence such as drug delivery devices with cloud connectivity to support patients’ therapeutic compliance and correct, regular medication intake. The development revolves around single-use versus multi-use delivery devices, and connected add-ons versus integrated connectivity. At the same time, counterfeit, adulterated and diverted products have become more widespread. PSI (Pharmaceutical Security Institute) reveals a 38% increase of fake products between 2020 to 2021. Pharmaceutical manufacturers are thus looking for reliable proof of authenticity, also for patients and end users. NFC enables easy (unambigious) authentication using smartphones, and brings much more security than an optical code, preventing mass copy and cloning activities.
AR: I can only emphasize the special importance of medication at home. Especially for chronic diseases, there are many new therapies being launched on the market that are no longer delivered at a doctor’s office or hospital. That results in a growing need to support patients to ensure successful therapies. It starts with a simple means of providing information about the use of a product, keeping a digital diary, and checking the authenticity of certain high-end drugs. However, that can only be achieved if such support is readily accessible for and easy to use by the patient. That is possible via NFC in combination with a smartphone or via smart devices that automate processes by means of an integrated NFC reader and inserted tagged consumables.
You have launched new ICs (microchips) with security features on the market—which products are they and what are their benefits?
SKK: NXP’s certified NTAG® 424 DNA chip product line offers advanced NFC security features for particularly high protection: AES(Advanced Encryption Standard)-128 cryptography and web-based SUN (Secure Unique NFC) message authentication for dynamic, secured NFC messages upon every NFC phone readout, in order to prevent mass copying. For an automated offline authentication in a device, a mutual authentication mechanism can ensure only an authenticated reader can access sensitive tag data, protecting it from unauthorized access or modification. For privacy, use of encrypted or random tag identifiers can be considered. NXP’s recently launched single-chip NFC microcontroller PN7642 offers a high integration level and small footprint for adding connectivity, processing and security in even small drug delivery devices. Manufacturers are thus provided with proof of authenticity to detect counterfeits. As a result, they also gain greater transparency about the products’ utilization on the market, and about irregular flows and other fraud of goods.
FV: For RAIN-RFID solutions, NXP offers UCODE® DNA TRACK: UHF tags with cryptographic security and long reading range of up to about ten meters, depending on the environment and readers used. It enables authentication at unit level or box/pallet level during automatic inventory management and ensures security within the supply chain.
AR: Particularly for such products, the realization of these authenticity features and their integration in customer-specific labels is very important. This ranges from a secure production environment all the way to cryptographic programming that we can offer as a specialized label manufacturer.
What are the latest developments in terms of your new sensor ICs, in other words sensors in the form of microchips?
SKK:Our goal is to develop technologies that make connected products more secure, intelligent, and efficient, using connectivity, condition sensing, and data processing on the respective device as well as in the cloud. Our NTAG 22x DNA StatusDetect Tags with capacitive structures have a simple passive sensing capability enabling measurement of a capacitance change. Upon NFC readout, such tag can generate a tamper-sensing value when its configured limits are exceeded, to provide a first-open status information. Or, the sensor tag captures a capacitive change of a specific ambient condition when interrogated, that can be further interpreted by a software application. This can be used for measuring the fill level of liquid containers, or to capture mechanical movements in medical devices, for monitoring medication intake, or for proof of compliance in clinical trials. In addition, our NTAG SmartSensor (NHS3100) offers a semi-active sensor solution (requires a battery for continuous monitoring, editor’s note) with an integrated microcontroller, a built-in temperature sensor and large memory for storing data. This sensor can be used on smart blister packs for tracking pill removal or cold chain monitoring of temperature-sensitive drugs.
COVID-19 and other factors have had a major impact on the supply chains, especially with semiconductor products—how do you assess the current and future situation?
FV:We continue to see a growing demand for RFID/NFC technologies. At NXP, we have taken various steps to further expand the capacities with manufacturing partners and in our own production facilities. In addition, we are extending our testing and packaging activities to reduce supply bottlenecks and improve lead times. We are in close contact with our customers for early coordination of forecasts and demand to ensure their supply. This transparency assists us in capacity planning and reliable order fulfillment.
What are the most important developments in recent and the coming years in terms of RFID use in the healthcare sector?
SKK:User safety and ease of use of medical devices is becoming increasingly important. More and more patients benefit from smart, connected care at home, e.g. to improve adherence, whilst hospitals benefit from error reduction through automation. A device such as an injector or infusion pump equipped with an NFC reader can automatically read the data of inserted consumables equipped with an NFC tag. Stored chip data can prevent the utilization of counterfeit, wrong or expired medicines, and the reuse of empty consumables. In addition, sensory feedback can help avoid dosing or application errors. This is because valuable context data regarding first opening, fill level, temperature and more can be captured. Secure offline authentication is possible with cryptographically secured consumable labels and readers with secure key storage. Personal devices make use of the increasing smartphone ownership, e.g. by sending data via BLE directly to a patient’s phone, for processing in an App or transfer to a cloud-hosted database. Key information such as dosage, date and time can be captured and tracked through automated features, and notifications can help remind patients when the next dose it due.
How do you rate the collaboration with Schreiner Group?
FV: Schreiner Group is an important partner that as an innovation driver finds new solutions for the challenges of the relevant industry—often by using the latest IC technologies from NXP, such as tamper-evident security labels combining digital and mechanical features. The company has an in-house design laboratory to facilitate the development of new prototypes and design-ins such as printed antennas and sophisticated embedded designs. In addition, Schreiner Group has extensive expertise in the field of security printing for the deployment of cryptographic authentication ICs including secure tag encoding and trust provisioning, on both NFC and RAIN-RFID tags. Its comprehensive knowledge on industry regulations and compliance issues helps support customers in approval processes.
RFID/NFC
RFID (Radio Frequency IDentification) is a technology for contactless data exchange. NFC (Near Field Communication) as a subset is tailored to smartphones etc.
RAIN
RAIN (RAdio-Frequency-IdenificatioN) is an industry alliance to promote the utilization of UHF-RFID. RAIN certifies UHF-RFID tags in terms of their quality.
UnitVisID Alliance
The UnitVisID Alliance is an industry alliance that establishes healthcare industry standards for RFID-tagged medicines.