Digital Medication Adherence Monitoring: Challenges, Trends, and Solutions
SCHREINER MEDIPHARM
Digital Medication Adherence Monitoring: Challenges, Trends, and Solutions
With their specialized expertise, Schreiner MediPharm and AARDEX Group complement each other perfectly, and with their smart solution for digital medication adherence monitoring, are pursuing the goal of enhancing patient compliance especially in clinical trials. Non-adherence to the prescribed dosage regimen by patients participating in a trial may have severe consequences—such as inadequately documented efficacy of the medication or underestimated frequency of side effects. Stefan Wiedemann from Schreiner MediPharm and Bernard Vrijens from AARDEX Group talk about the capabilities of the digital Medication Adherence Monitoring solution and why it meets current and future trends.
How does the smart digital adherence monitoring solution from Schreiner MediPharm and AARDEX Group work?
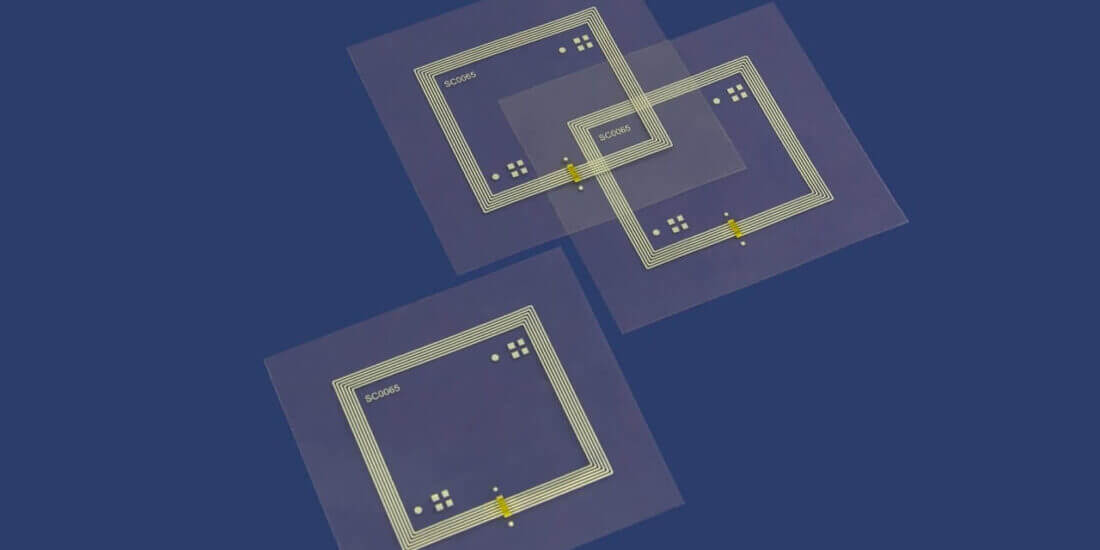
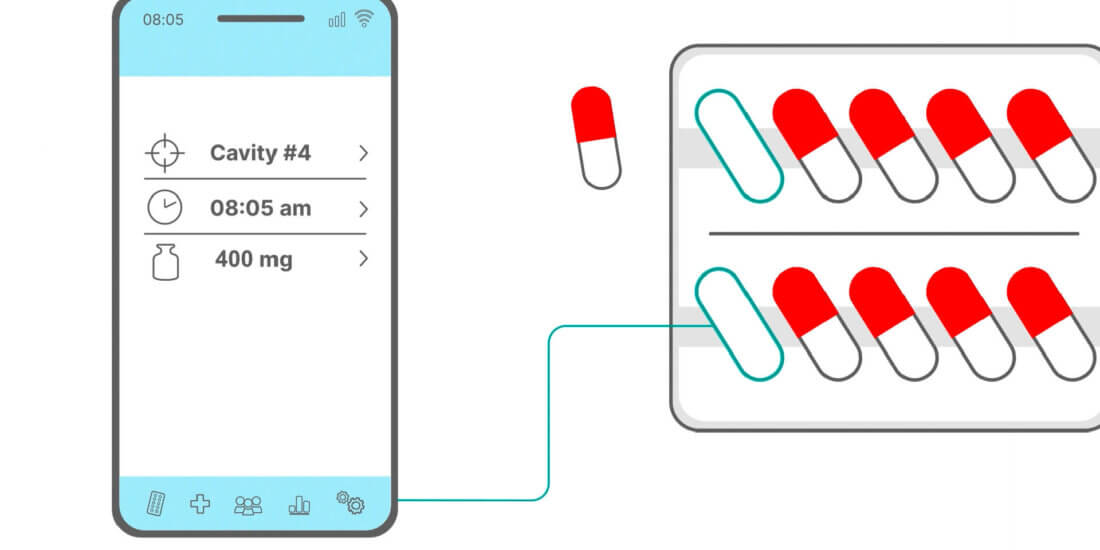
Stefan Wiedemann (SW): Simply put, this is a smart form of pharmaceutical packaging combined with matching software. Blister packs for tablets are equipped with integrated electronics and sensor technology. Patients push their tablets out of the cavities as usual. The electronics are used to generate real-time data, such as time of removal, cavity, and dose, automatically store the data in the pack, and transmit it to a database via a smartphone app or reader. Specialized software subsequently serves to analyze the data.
How can digital adherence monitoring tools enhance patient compliance? Bernard Vrijens (BV): Digital Adherence Monitoring Tools can have a tangible impact on patient adherence to medications. But before we dive into the specifics of that, let’s look at the ramifications of poor adherence for the research industry. The stakes are high, both for drug developers and patients. When patients don’t take their medicine as prescribed, it can have life-altering or even life-threatening consequences.
Traditional, non-digital methods for monitoring adherence, such as pill count and self-report, do not consider such intricacies. They are subjective, open to bias, and merely provide a snapshot of medicine-taking behavior. Analogue methods also place an additional burden on patients and site staff, detracting from their utility. Digital adherence monitoring is different. It offers a holistic framework that can inform individualized intervention, even in therapeutic areas with notoriously high poor adherence rates, Oncology, and Psychiatry for example. What’s more, this type of approach is frictionless for the patients—all it asks of them is to take their medication.
What are possible drawbacks/challenges when it comes to digital adherence monitoring solutions and how can they be overcome?
BV: Historically, medication adherence has been the elephant in the room. And there’s a myth that drives this. Many know that poor adherence is prevalent in practice, but believe that it’s good in trials. We know that not to be true. Adherence is poor across the board. What’s more, some sponsors may not even realize that they have a non-adherence issue in their trials, and this is simply because what isn’t measured can’t be managed. This is a challenge—one that we can address quite easily in fact. Our approach to managing adherence is evidence-backed. Another challenge for sponsors is the change management process and the perceived complexity of implementing such solutions. This challenge isn’t exclusive to adherence management technology. It’s typical in industries that are very highly regulated.
SW: The complete solution for digital therapy control in clinical trials that we’ve developed can overcome exactly these challenges. It enables precise compliance with the requirements and dosage regimens for drug development according to the proposed clinical trial protocols of the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Likewise, our digital tool can reveal structural deficits and collect important data about patient behavior. Such data can subsequently be used for marketing strategies.
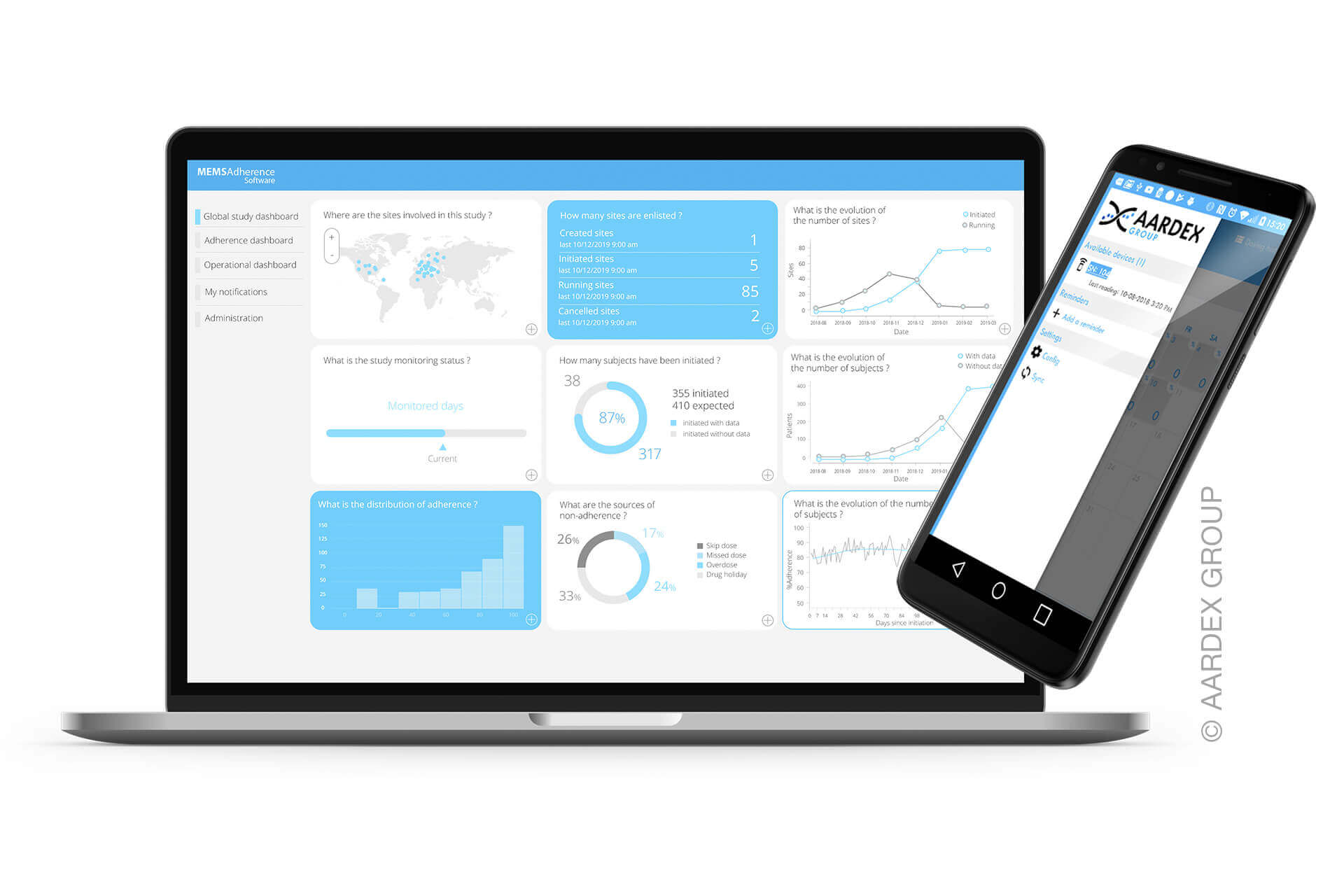
What is AARDEX Group’s core competency and what is special about AARDEX MEMS® adherence software?
BV: What is measured can be managed, and on this front, AARDEX Group delivers. We’ve purposely expanded our ecosystem of partners to ensure that we have a device that can capture adherence behaviors—regardless of the route of administration. But collecting the data is only one side of the coin. The true value in MEMS AS® software is the statistical algorithms and behavioral analysis that underpin the solution. The inherent data processing capabilities negate the need for additional statistical analyses as the tool does it all automatically. Patient adherence analytics are conveniently presented in meaningful dashboard visualizations that can be interpreted at the patient, site, and study levels. Having access to readily available dashboards like these gives investigators the information they need to drive risk stratification and prevention. What’s more, the product is platform-agnostic and can be integrated with Interactive Response Technology (IRT), Electronic Data Capture (EDC), and Decentralized Clinical Trials (DCT).
What is the key benefit for AARDEX Group in cooperating with Schreiner MediPharm?
BV: Partnering with Schreiner MediPharm has allowed us to further reinforce our ecosystem with a solid partner for smart blisters. Together, with our common goal of enhancing the efficiency of drug development, by offering data generating packages and end-to-end solutions to manage medication adherence digitally, we will continue to thrive as the industry continues the shift to digital. What’s more, Schreiner MediPharm is a key player in this space, with solutions proven to deliver quality and high standards.
In your view, what are the key trends in Clinical Trial Management?
BV: For decades, the research industry has relied on the traditional trial model, where the majority of the trials conducted was built around sites, necessitating the need for in-person visits and monitoring. This was largely due to the fact that many sponsors were still using paper-based systems. However, when the pandemic hit, imposing restrictions on movement to reduce infections, the industry was pushed into a state of flux. While some sponsors had to halt trials, others embraced technology to ensure trial continuity. During this time, the industry truly realized the benefits of remote monitoring, not just for those conducting research but also for the participants. As a result, the use of remote monitoring has skyrocketed, prompting the FDA to create and publish draft guidance for the use of digital health technology in clinical investigations. I believe that the advent of this draft guidance will further cement Source Data Verification (SDV) for what it is, antiquated, and that the use of digital health technology and remote monitoring technology trials will rise rapidly.
How can digital adherence monitoring tools enhance patient compliance and how can they meet current and future trends?
BV: The utilization of Digital Health Technologies (DHT) is rising rapidly in clinical research. When talking about the decentralization of trials, David Burrow, the director of the FDA Office of Scientific Investigations, was quoted stating that they were “here to stay”. This, combined with the recent FDA draft DHT guidance, will serve as catalysts for the industry’s ongoing shift to digital. However, with fewer or no site visits on the table, this model could present an issue for sponsors that rely on traditional methods of measuring adherence, blood sampling, etc., Digital Adherence Monitoring bridges the gap by continuously monitoring patient medication-taking behaviors remotely.
What are the tangible benefits of the partnership between Schreiner MediPharm and AARDEX Group for pharmaceutical manufacturers?
SW: In our cooperation, we combine Schreiner MediPharm’s in-depth expertise in printing and packaging technology with AARDEX Group’s IT intelligence. We provide pharmaceutical manufacturers or CMOs with a wallet including printed electronics and all they have to do is integrate the blister. Their production processes remain unchanged. The flexibility we’re able to offer them is a major benefit. Due to roll-to-roll processing, our technology can be scaled from small volumes all the way to mass manufacturing. As a result, pharmaceutical manufacturers can fully exploit the potential of the digital adherence monitoring solution in clinical trials as well as use it in the commercial context.
AARDEX Group
Located in Belgium, Switzerland and the U.S., AARDEX Group develops and markets digital solutions to measure, analyze and manage medication adherence in clinical trials, research settings, and professional healthcare systems. AARDEX Group is the central actor of a complete ecosystem that combines its MEMS® Adherence Software with a wide range of smart packages and devices that measure patient adherence to all routes of drug administration. AARDEX Group’s vision is to continuously innovate in data-driven medication adherence solutions to enhance digital therapeutics and patient empowerment. www.aardexgroup.com