Cosmeceuticals: Syringe Labels for Beauty
SCHREINER MEDIPHARM
Cosmeceuticals: Syringe Labels for Beauty
Beauty is a subject that has always played a major role in the world’s diverse cultures over the centuries. Today, the question of personal beauty has greater relevance than ever before and is reinforced by the power of pictures and social media: In 2019, 25 million cosmetic procedures were performed worldwide — and the trend is rising. More than 4.3 million of them were wrinkle treatments with hyaluronic acid injections. Plastic syringes are increasingly used to administer them in cosmetic medicine. For ease of use, optimal marking, and reliable tamper protection, Schreiner MediPharm offers a wide variety of labels with value-adding functions for cosmeceuticals syringes.
Cosmetic surgery is a growth market. More and more people are trying to counteract the natural skin aging process by means of aesthetic injections. For facial wrinkle treatments, injections of so-called dermal fillers are used. They include hyaluronic acid, a gel with particularly high viscosity. The thinner the syringe used for the injection, the smaller the physical exertion required by the cosmetic surgeon. Polymer syringes have a number of advantages over glass syringes, such as higher break resistance, lower weight, and greater custom-design flexibility. However, prefilled COC syringes have limited migration properties. In addition, tamper protection poses a challenge.
Labels with value-adding functions optimize the polymer syringes that are frequently used in the field of cosmetic medicine.
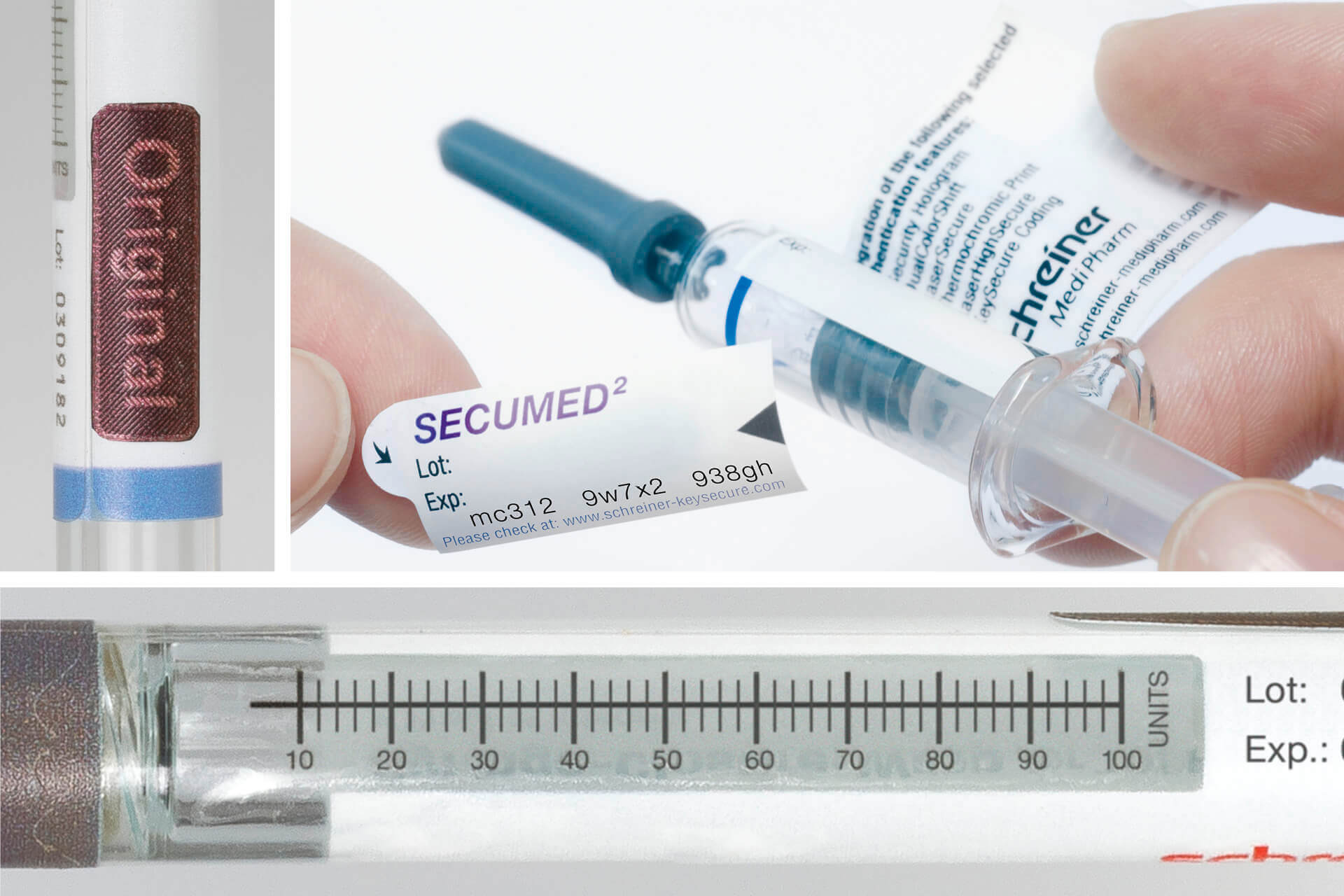
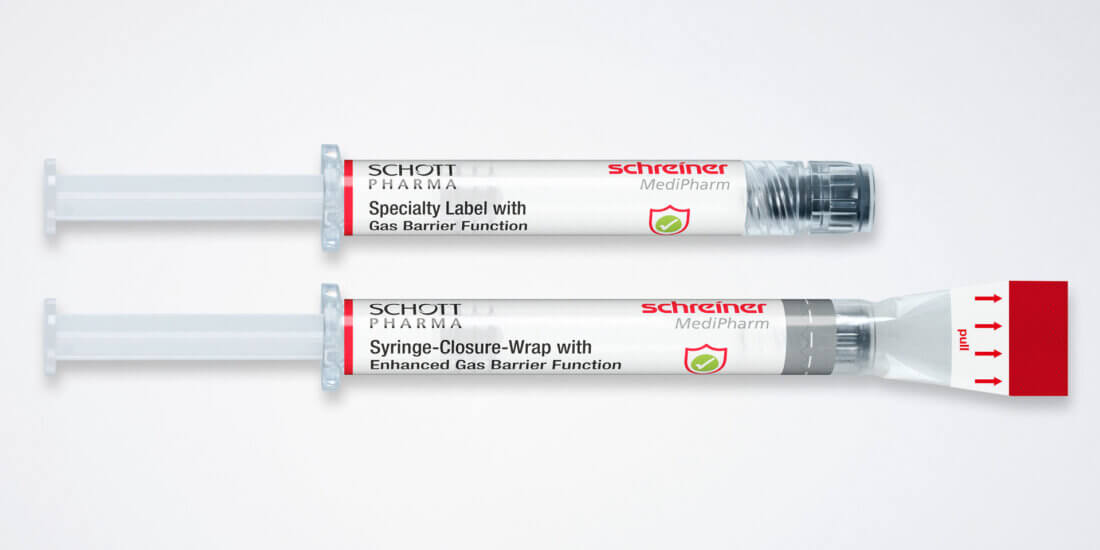
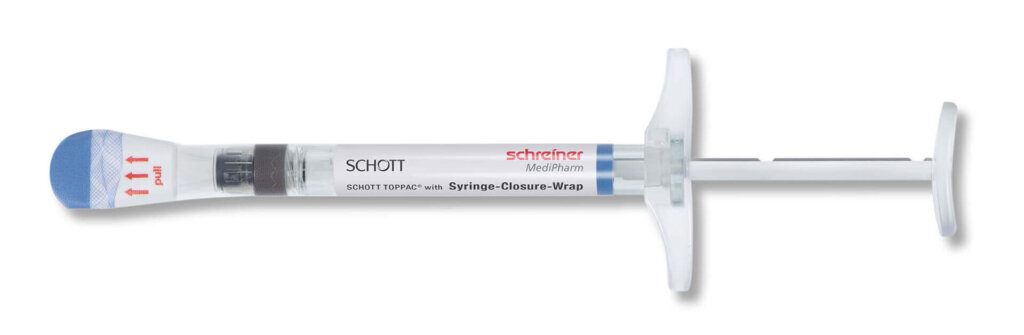
For cosmeceuticals syringes, Schreiner MediPharm offers a wide variety of customized functional labels that simplify handling, enhance tamper protection, and compensate for material deficits. The label design, for instance, can incorporate precision graduations such as milliliter markings and dosing units for exact injection dosing. Tamper-evident and first-opening indication solutions such as the new Syringe-Closure-Wrap for sealing prefilled syringes protect the integrity of the syringe. For product and brand protection, overt, covert, and digital authentication features can be integrated into the label. They enable authentication along the supply chain—from laypersons to informed experts—depending on the security level.
In addition, Schreiner MediPharm offers suitable solutions such as the multi-layered Pharma-Comb ILSC label for attaching comprehensive product information to cosmeceuticals syringes with their narrow syringe radii. The label is wrapped tightly around the syringe, can be opened and closed again easily, and is additionally equipped with detachable documentation labels.
Low-migration labels can be adapted precisely to the respective application using pre-qualified materials, which clearly minimize the risk of migration that polymer syringes entail. Schreiner MediPharm can draw on comprehensive outcomes of studies involving low-migration adhesives, materials, and inks, and provide customer-specific advice.
The functional, customized syringe labels for cosmeceuticals provide manufacturers of cosmetic medicine products with solutions ensuring process reliability, flexibility, and smooth production processing, while supporting injection comfort and safety, and protecting patients.