Booklet-Labels for Clinical Trials: Efficient and Flexible
SCHREINER MEDIPHARM
Booklet-Labels for Clinical Trials: Efficient and Flexible
Ample information–quickly available: Schreiner MediPharm’s expert team for Clinical Trial Supplies (CTS) enables customized Booklet-Labels for a wide variety of uses. Thanks to a specialized process center, validated solutions are created according to the exacting quality standards of the pharmaceutical industry.
Clinical trials are often conducted on an international scale and call for utmost precision as well as efficient and safe processes. The investigational drugs must be reliably marked and provided with all the key information in several languages. Flexibility is crucial in order to enable short-term quantity adjustments in different countries.
The Solution: Booklet-Labels
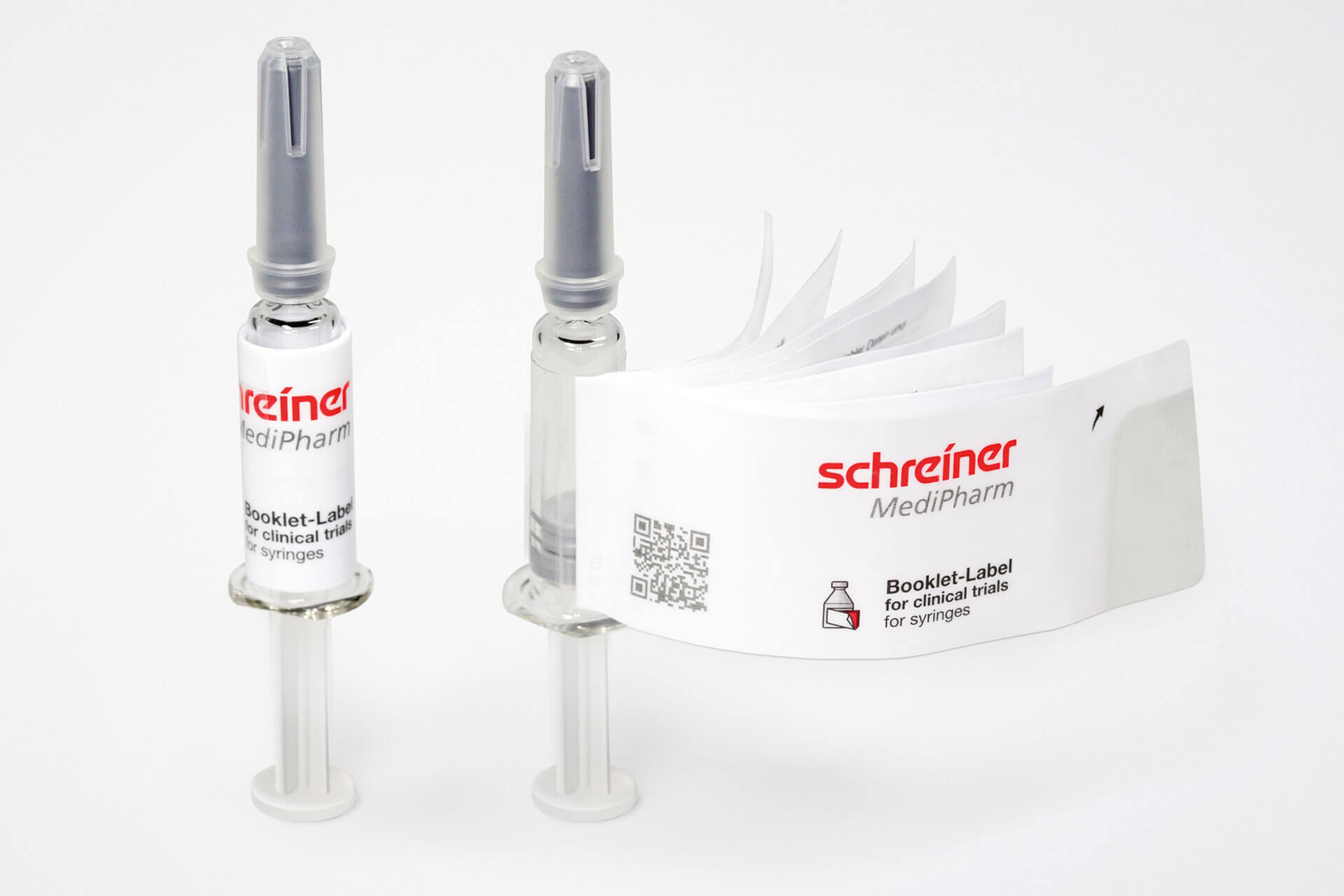
Booklet-Labels are ideally suited for clinical trials because they have many pages and offer ample space for extensive text in several languages. Schreiner MediPharm’s CTS team develops customized Booklet-Labels reliably adhering to various container shapes and packaging, whether they are vials, syringes, blisters, pens/autoinjectors, tubes, boxes, or plastic containers.
Due to the use of very thin paper, the material structure of the Booklet-Labels is particularly flexible and suitable even for small containers with narrow radii. Value-adding functions such as hangers for infusion bottles, detachable documentation labels, RFID/NFC chips for interactive applications, or security features can be integrated. The combination with opaque films ensures reliable blinding. Special adhesives and materials additionally enable uses even at sub-zero temperatures. Increasingly, the sustainability of the solutions is moving into focus as well.
Efficient Production and Specialized Services
Schreiner MediPharm’s CTS team advises and supports customers individually across all trial stages, while achieving the fastest possible delivery times. Standardized workflows, materials, and testing processes ensure efficient and process-compliant production of the Booklet-Labels in a specialized process center. That’s where special services such as validated post printing of variable and randomized data are available as well. In addition, to optimize processes, Booklet-Labels can be pre-produced and placed into intermediate storage for variable post printing on short notice as needed. Regardless of the requirements, the CTS team will find a solution!
Further information about the wide range of solutions for clinical trials can be found on our website:
https://www.schreiner-group.com/en/products/clinical-trials