A Double Dose of Safety: Needle-Trap Secu With First-Opening Indication
SCHREINER MEDIPHARM
A Double Dose of Safety: Needle-Trap Secu With First-Opening Indication
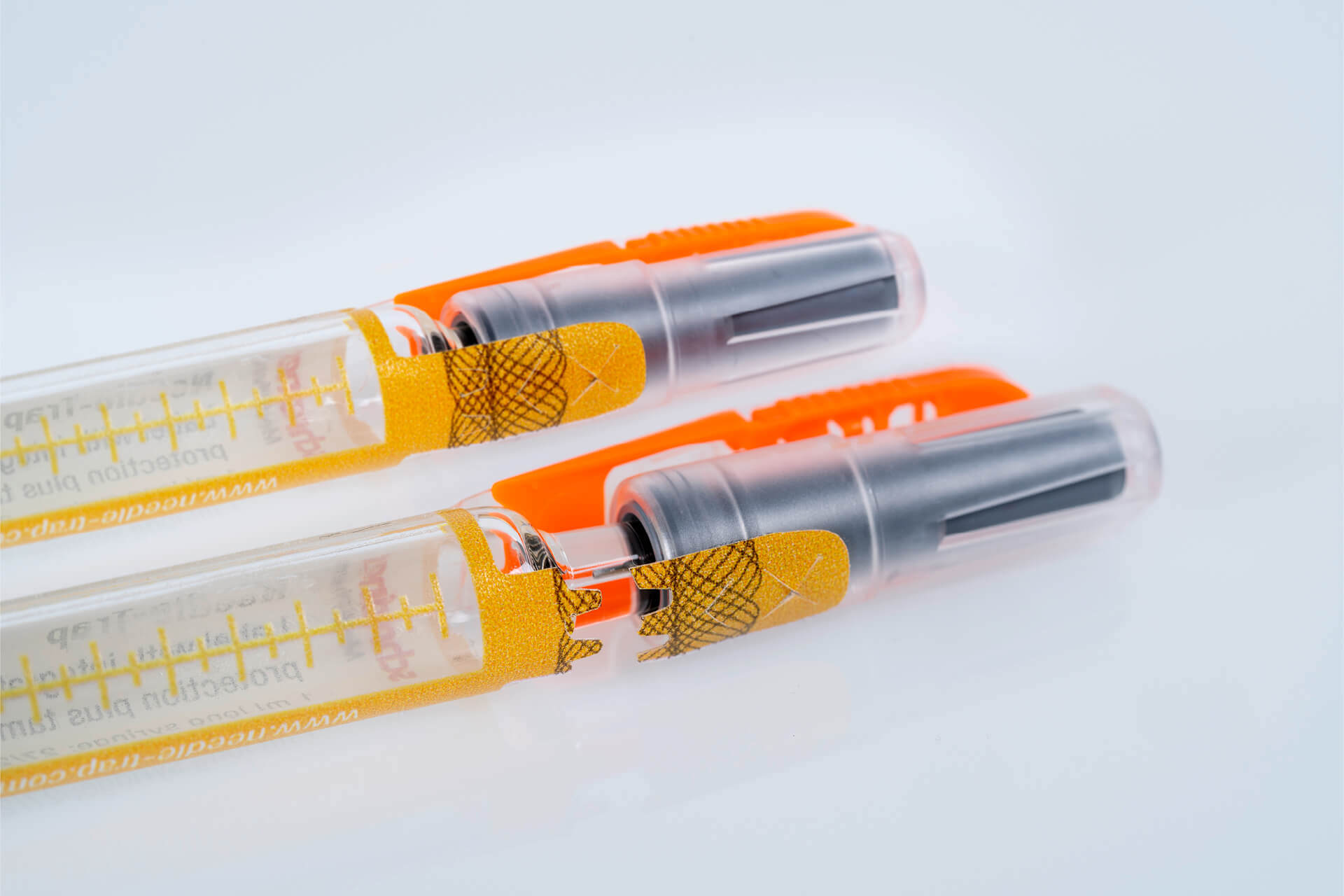
More than 1.5 billion units produced and recognized with ten international awards: That has been the success story of the Needle-Trap needle protection system from Schreiner MediPharm since its market launch in 2009. Now the label with an integrated needle trap for protection against needlestick injuries has been complemented by a new feature: A security seal reliably indicates if the syringe has previously been opened. Needle-Trap Secu is a clever combination of needle and product protection that provides users with a double dose of safety and protects the integrity of the prefilled syringe that is important for the pharmaceutical manufacturer.
A secure supply chain and the integrity of medicine packaging are essential for pharmaceutical manufacturers. Regulations such as the EU Falsified Medicines Directive support that need but only address secondary packaging. Primary containers, however, require more specialized solutions for tamper evidence and first-opening indication. Due to its integrated plastic trap, Needle-Trap poses special challenges concerning first-opening indication. Smooth processing of the label on dispensing systems in pharmaceutical manufacturing environments and ease of use in healthcare settings must be considered.
Needle-Trap Secu with first-opening indication is a clever combination of needle and product protection.
Easy Process Integration–Safe Use
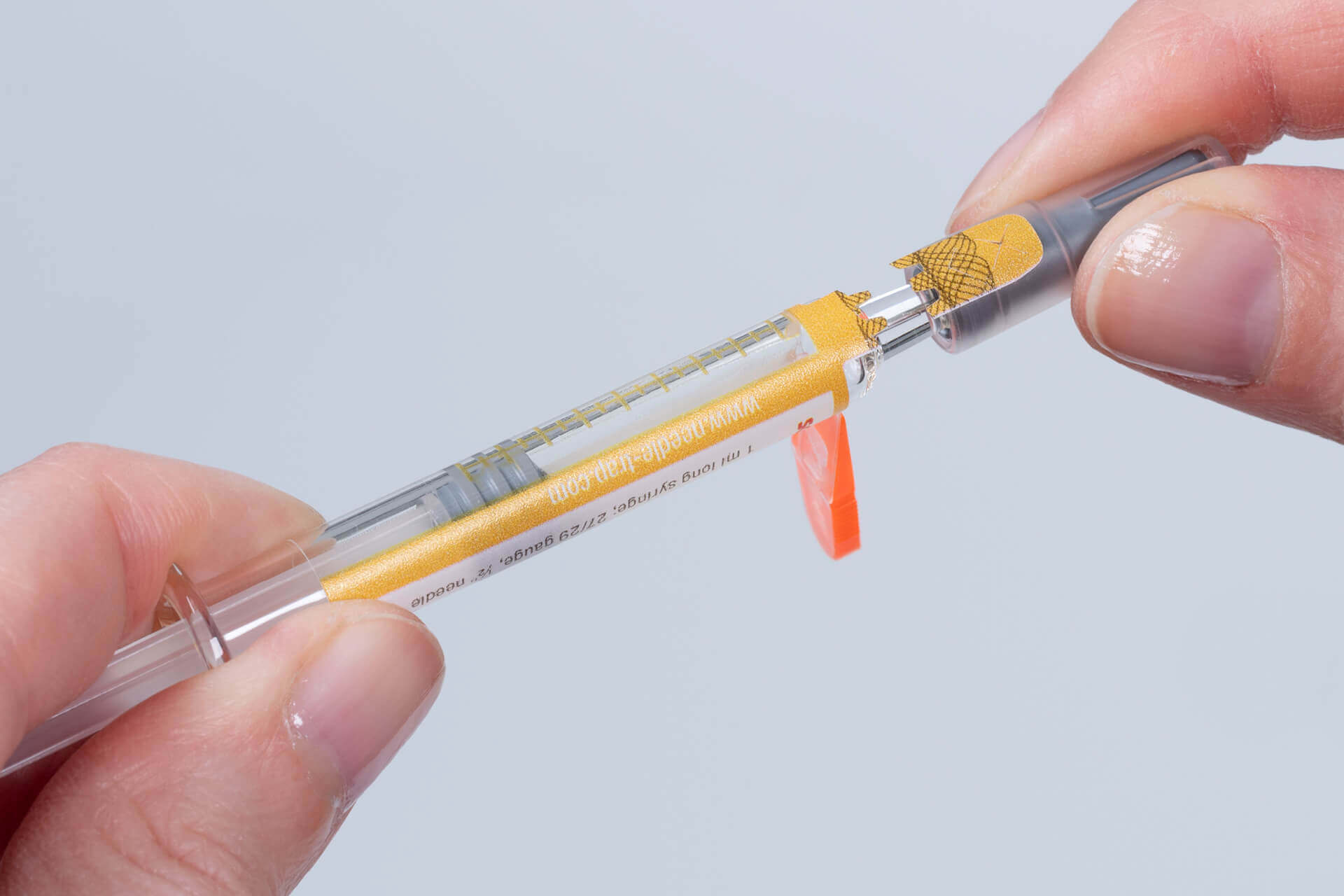
Needle-Trap Secu features a closure seal integrated into the syringe protection label with a tab ending on the syringe cap. Prior to the injection the needle trap is folded sideways as usual. While the cap is being pulled off a perforation triggers the activation of the first-opening seal. Security cuts additionally prevent undetected removal of the seal. The closure seal can also be complemented by overt authentication features, such as a guilloche, or covert security features for authenticity verification. Pharmaceutical manufacturers are provided with a multifunctional and cost-efficient solution that can be integrated easily into existing production processes and enhances product and patient safety. The integrated seal secures the integrity of the prefilled syringes on unit level until the drug is administered. Thus, it eliminates the need for a blister pack, reducing space and waste. Healthcare staff benefit from the efficient and reliable needle protection as well as from convenient first-opening indication that is irreversible and detectable at first glance.
Needle-Trap Facts
Needle-Trap is the only label-based needle protection system worldwide. It meets the requirements of EU Directive 2010/32/EU and DIN EN ISO 23908 for protection against sharps and the U.S. NIOSH requirements for safe instruments. In addition, Needle-Trap has been awarded 510(k) Pre-Market Notification by the U.S. Food and Drug Administration (FDA) for marketing in the United States. The needle protection label can be integrated easily into existing pharmaceutical production processes and requires only minimal modifications of the application systems. It requires no secondary packaging adjustments, minimizes space requirements during transportation, storage, and disposal, and is thus particularly sustainable compared to conventional needle protection systems.