New Dress Code for Prefilled Syringes: Secure and Sustainable
SCHREINER MEDIPHARM
New Dress Code for Prefilled Syringes:
Secure and Sustainable
Decisively influencing a sustainable supply chain requires rethinking the design of packaging. To do so, three founding members of the Alliance to Zero have pooled their expertise: SCHOTT Pharma, Körber Pharma, and Schreiner MediPharm. As representatives of the three partnering companies, Christoph Zauner, Lidia Smith, and Nadine Lampka explain the novel approach in an interview.
What are the key benefits of prefilled syringes compared to vials?
Christoph Zauner (CZ): Prefilled syringes are immediately ready for use and contain the correct dose. That enhances the efficiency of administering medicines and helps avoid medication errors. In addition, contaminations of the active ingredient during the preparation of the injection are avoided and drug wastage in the event of unneeded doses is reduced.
Aside from product safety, sustainability and costs play an important role in the pharmaceutical supply chain. Where do you see further optimization potential in packaging design?
Nadine Lampka (NL): To minimize the environmental impact and to maximize efficiency, it is necessary to question traditional packaging methods. For instance, what is the benefit of a blister pack? It is currently the standard for prefilled syringes because it comes with a variety of features. It serves as first-opening indication, protects the syringe against mechanical stress and contaminations, or can provide oxygen or UV protection. However, it also entails disadvantages in terms of sustainability, costs, and process complexity.
How can blisters be dispensed with?
Lidia Smith (LS): Due to a novel “dress code” for prefilled syringes, we can transfer the functionalities of the blister pack to the primary container, achieve additional benefits, and, in combination with a special type of secondary cardboard packaging, become sustainable.
What does the new dress code look like and what are the various elements?
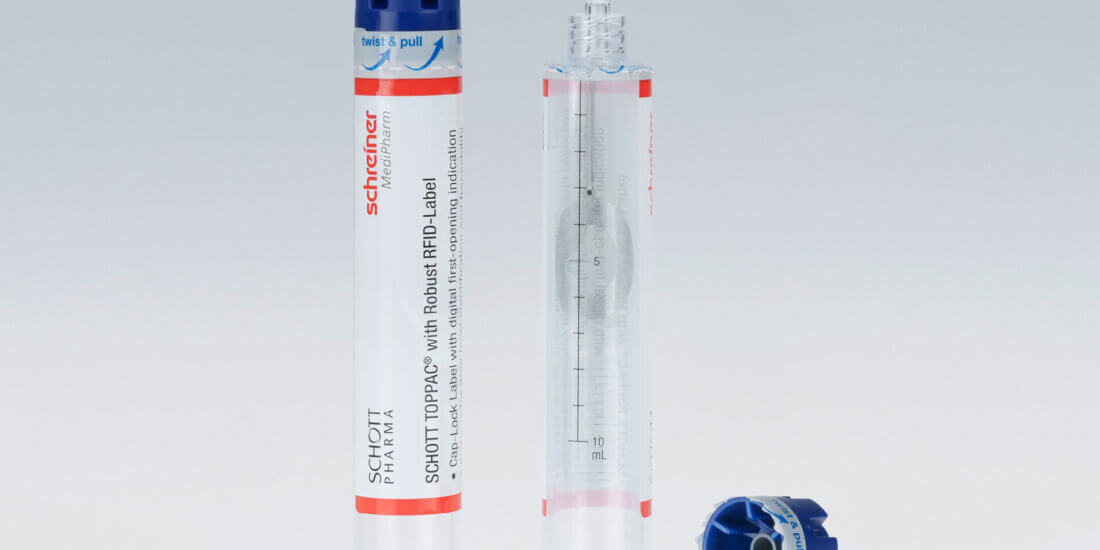
CZ: For our SCHOTT TOPPAC® infuse COC syringe, there will be a new cap design covering a variety of syringe sizes. The syringe is delivered in ready-to-fill form, requiring no cap assembly after filling. Thanks to a pre-tested system of the syringe and label, pharmaceutical manufacturers are provided with a solution ensuring a reliable process.
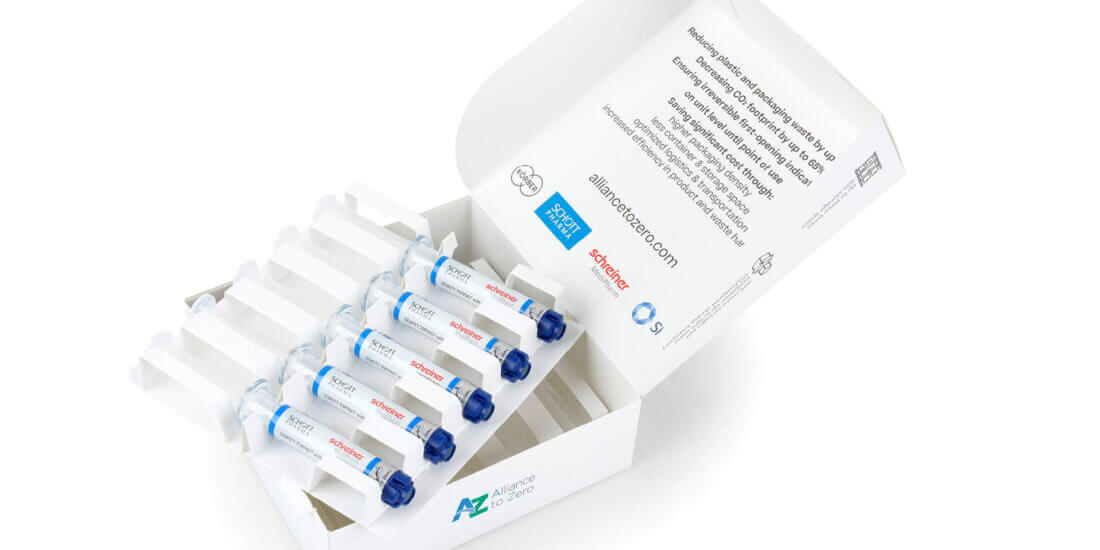
NL: The label is the part where we come into play. Because the specialty label wraps around the syringe and cap like a second skin it serves as an irreversible first-opening indication and performs the functions of the blister up until final use. The integration of a gas barrier is possible as well as customized UV and light protection. The enlarged label area also provides more space for product information or drug color codes. Additionally, digital first-opening indication and automatic RFID-based tracking on unit level can be integrated.
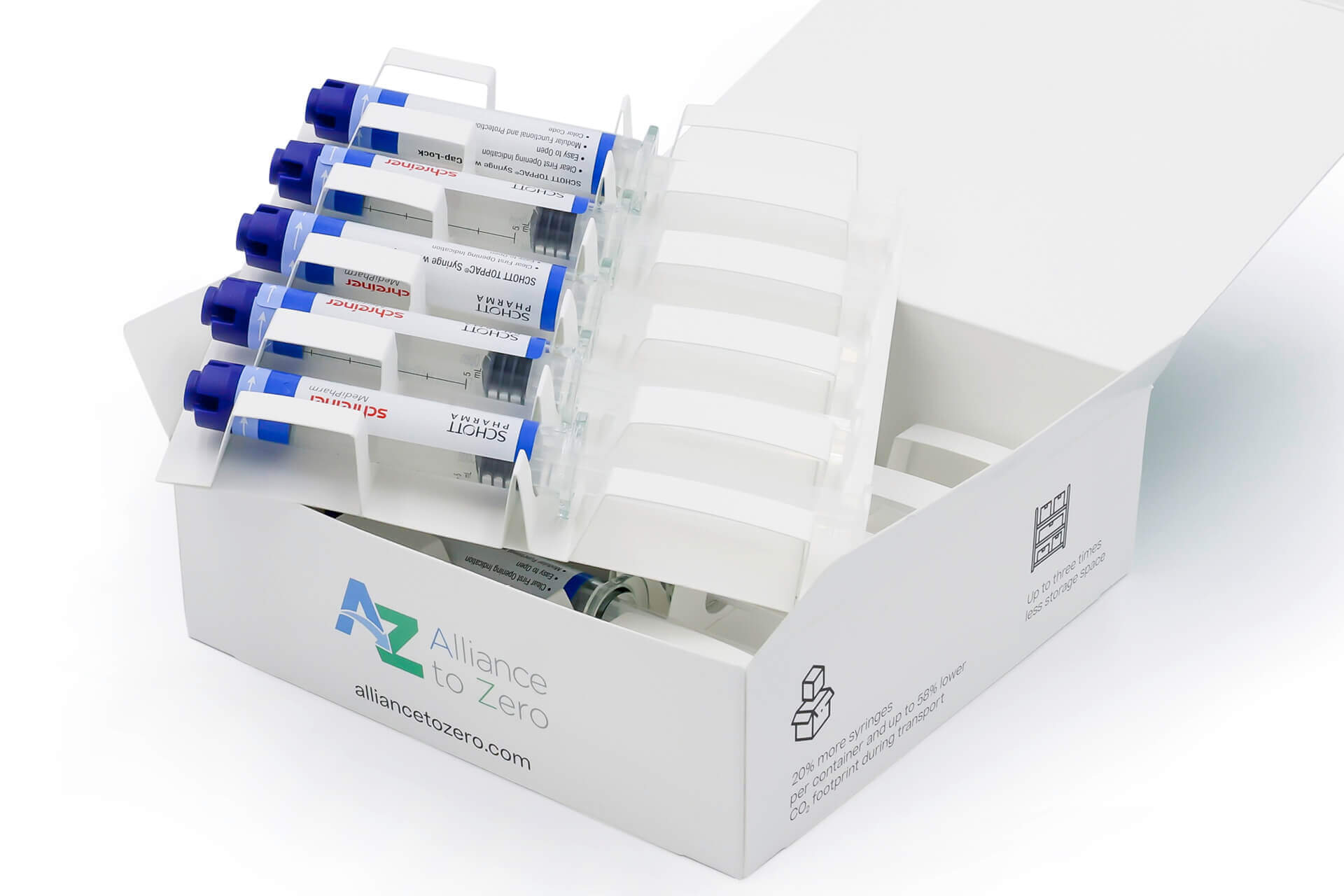
LS: Due to the combination of a COC syringe with a special cap and a functional label with sealing function, the prefilled syringes can be packaged in a volume-optimized cardboard box consisting of 100 percent mono-material. Our top-load boxes with integrated dividers for reliable retention of the product are compact and eco-friendly. The system of the prefilled syringe featuring a new cap design plus the label and cardboard box has already successfully passed an ISTA (= International Safe Transit Association) 3A transportation test.
What are the benefits of the new dress code along the value chain?
NL: The benefits are enormous, encompassing optimized and more cost-efficient packaging and distribution processes in pharmaceutical production, clearly less space required during transportation and storage, and more efficient handling processes of the prefilled syringe in hospital settings up to and including disposal, with clearly reduced and more eco-friendly packaging waste. Last, but not least of course, increased patient safety as well.
Are concrete figures available in terms of optimization potential?
CZ: Using the example of the 5 ml SCHOTT TOPPAC® infuse, we calculated how many syringes with and without blisters can be packaged per pallet. Thanks to the 25-percent reduction in packaging size, an additional 1,260 syringes can be shipped per pallet, equating to 126 additional boxes of 10 syringes each. The more compact packaging size impacts the container space as well. In the case of 10 million syringes, 16 fewer containers are needed, which naturally means considerable logistics cost savings too.
LS: In this specific case, avoidance of the blister also results in major, 80-percent reductions of plastic materials and plastic waste. Assuming that 1 kg of plastics corresponds to twice the amount of CO2, 68,200-kg reductions of CO2 can be achieved in a single haul of 10 million 5 ml syringes from Hamburg to New York, and adding the logistics savings, 87,400 kg of CO2 in total.
NL: Impressive figures emphasizing the value of our joint approach of pursuing new pathways along the supply chain and significantly reducing the carbon footprint in terms of a sustainable pharmaceutical supply chain.